ScheBo Biotech AG:
High-tech Biotechnology fighting cancer
An innovative biotech company from Germany that focus on Quality, Clinically Relevant Innovation and Simplicity, proudly presents:
|
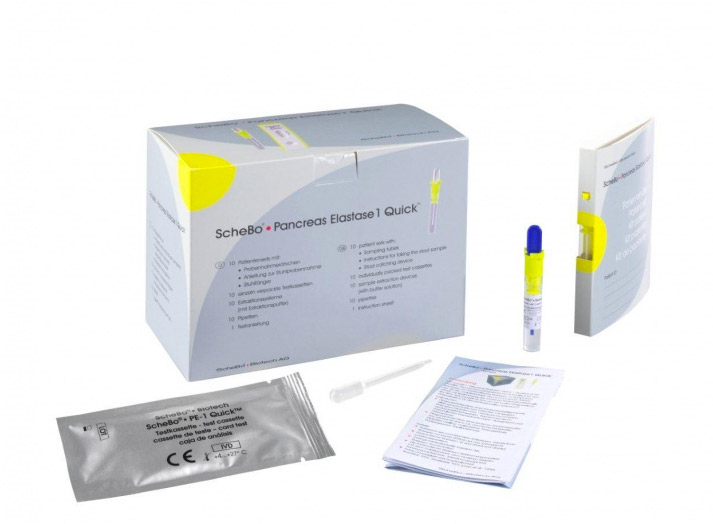
Schebo® Pancreas Elastase 1 Quick™
|
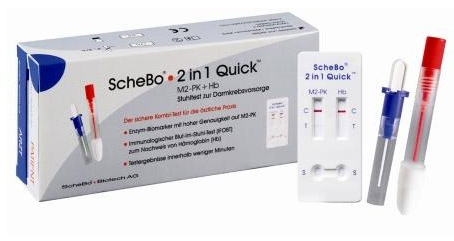
Schebo® 2-in 1 Quick™
|
|
Schebo® M2-PK Quick™
|
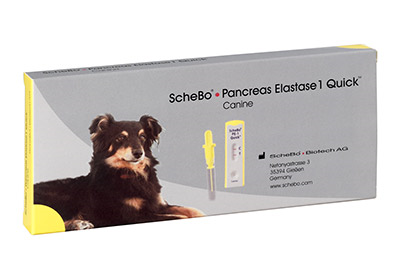
Schebo® Pancreatic Elastase 1 Quick™ - Canine
|
Tests available from Schebo Biotech AG:
Tests for human pancreatic elastase 1
Schebo® • Pancreatic Elastase 1™ Serum Test
Schebo® • Pancreatic Elastase 1™ Stool Test
Schebo® • Pancreatic Elastase 1 Quick Test
Highly sensitive and specific metabolic biomarker for colorectal cancer
Schebo® • M2-PK™ EDTA Plasma Test
Schebo® • M2-PK™ Stool Test
Schebo® • M2-PK Quick™ Test
Schebo® • 2 in 1 Quick™ Test
Multi-functional stool sample extraction system
Schebo®• Master Quick-Prep™
Non-radioactive pancreatic function test for dogs
Schebo® • Pancreas Elastase 1 Quick™ - Canine